


MADS® is currently in its final prototype phase and nearingreadiness for clinical testing and real-world patient treatment.
As the prototype nears completion, it will undergo rigorous testing to ensure it meets the functional requirements defined for the various components of MADS®.
Once the final prototype is fully developed, an extensive functional evaluation will be carried out in a bio-mechanical laboratory (in vitro).
This testing will provide precise data on the improved drainage performance of MADS® compared to existing technologies and methods.
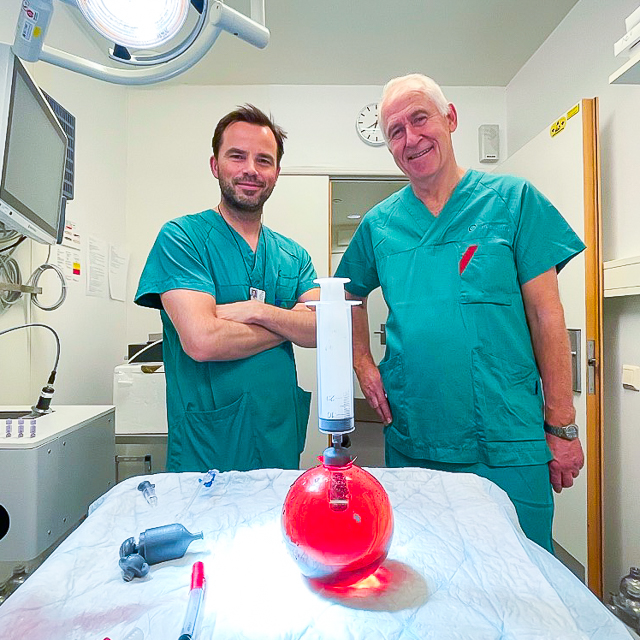
Following successful in vitro testing in the bio-mechanical lab, MADS® will be ready for clinical evaluation in real patient scenarios (in vivo).
These trials will be conducted in close collaboration with some of Norway’s leading surgical specialists at Rikshospitalet in Oslo, as well as cardiologists and nursing staff at both Stavanger University Hospital (SUS) and Rikshospitalet.

At Madsnor, we believe that advanced technology like MADS® empowers healthcare professionals to deliver high-precision treatment that protects patient health from day one.
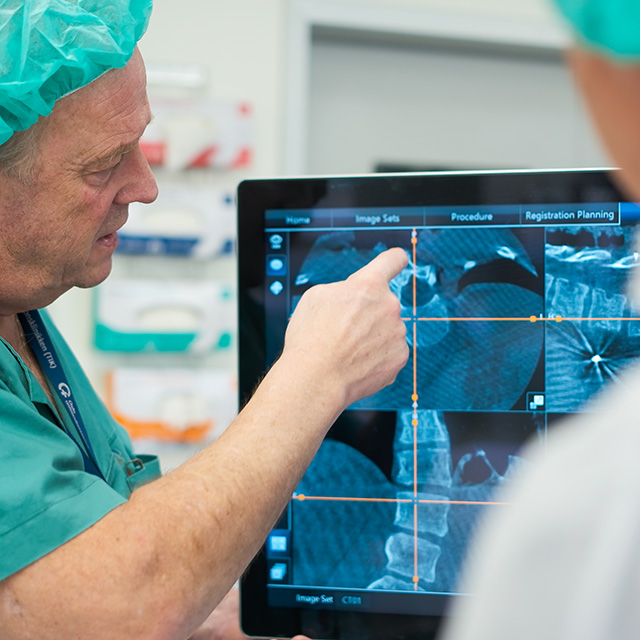
Initial tests on the first prototype have already demonstrated that MADS® delivers significantly enhanced performance compared to current drainage technologies.
The time required to completely drain a simulated abscess was reduced by 50%.
Additionally, MADS® enables automatic flushing of substantially larger volumes of cleansing fluid than what is currently achievable through manual methods. This innovation can reduce the workload for healthcare personnel and cut the average treatment duration in half — from 12 days to just 6 days of hospitalization.